“Any treatment given for coronavirus other than general supportive care, treatment for underlying conditions, and antibiotics for secondary bacterial complications, should currently be as part of a trial, where that is possible.”
Letter from Chief Medical Officers of Wales, Scotland, Northern Ireland, England to NHS Staff
Creation of the RECOVERY trial platform during the COVID-19 pandemic has been a milestone in clinical trial processes. The RECOVERY trial platform has been built on Richard Peto’s promotion since the 1970’s of large, simple trials using factorial designs to make more than two treatment comparisons (Peto et al. 1976; 1977; Peto 1978; Yusuf et al. 1984). The RECOVERY trial platform (hereafter ‘RECOVERY’) has extended that model with some novel design features, particularly a very rapid set up and recruitment process. RECOVERY has set new benchmarks for trial efficiency. Importantly, results obtained through RECOVERY have had a strong impact on clinical practice and clinical guidelines during the COVID-19 pandemic – a feat not achieved during any previous pandemic.
In parallel to RECOVERY, WHO has also created and coordinated a large multi-country trial platform with a similar protocol to RECOVERY. By the end of January 2021, WHO’s SOLIDARITY platform had enrolled over 14,000 patients in more than 400 hospitals in over 30 countries, and it has provided by far the most statistically precise estimate of the effects of remdesivir in COVID-19. Interim remdesivir results were published in December 2020 (WHO Solidarity Trial Consortium 2020) and the final analyses are expected to be published in summer 2021. At the end of January 2021, trial recruitment using the SOLIDARITY platform was paused to deal with several logistic difficulties, but recruitment is expected to resume during July 2021.
Although the important lessons resulting from the RECOVERY and SOLIDARITY trial platform experiences are similar (Tikkinen et al. 2020), in this commentary we focus on RECOVERY. In brief, the ‘Randomised Evaluation of COVID-19 therapy (RECOVERY)’ trial is a randomized, controlled, open-label, adaptive, trial platform enabling comparisons of a range of possible treatments with usual care in patients hospitalized with COVID-19. The initial four experimental arms were: (i) lopinavir-ritonavir (commonly used to treat HIV), (ii) low-dose dexamethasone, (iii) azithromycin (a commonly used antibiotic), and (iv) hydroxychloroquine. Using its adaptive design and successful recruitment, RECOVERY has also already assessed the effects of (v) aspirin, (vi) colchicine, (vii) convalescent plasma, (viii) Regeneron’s monoclonal antibody cocktail, and (ix) tocilizumab. As of 15 July 2021, RECOVERY is being used to test (x) baricitinib (an immunomodulatory drug used in rheumatoid arthritis), (xi) dimethyl fumarate (an immunomodulatory drug used in psoriasis and multiple sclerosis), and (xii) high-dose vs standard dose of corticosteroids.
The RECOVERY protocol is available for use by investigators everywhere to design their own randomized trials to help identify treatments for COVID-19 with important beneficial effects. https://www.isrctn.com/ISRCTN50189673
Some special features of RECOVERY include:
- Simplicity (use of ISIS-2 1988 protocol)
- Trial platform design, with 4 arms and a control group (2x size of the treatment arms)
- Rapid set up and recruitment – protocol in 2 days, first patient recruited 9 days later
Simplicity
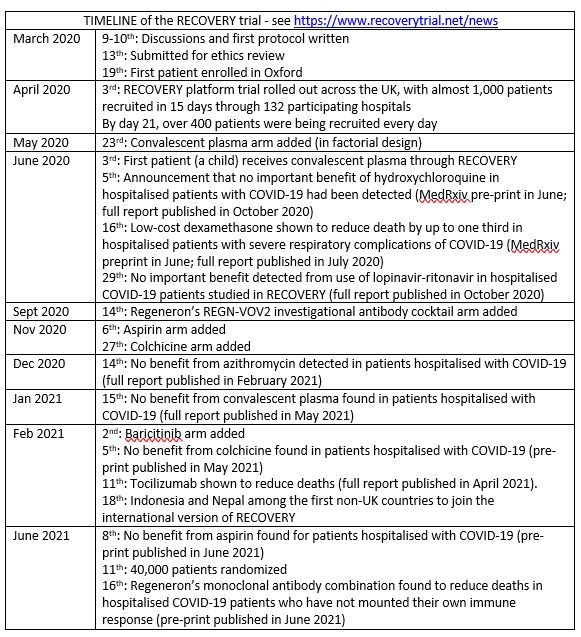
The protocol, written over two days (see Timeline box) was adapted from the large, simple, pragmatic trials exemplified by the ISIS trials (e.g., ISIS-2 1988) and the GISSI trials (e.g., GISSI 1986; Maggioni et al. 1990; Tognoni et al 2018) using broad, simple, inclusion criteria and minimising data collection to enable participation by COVID-19-stretched hospitals. The design of RECOVERY was based on ISIS-2 protocol – a large, simple, pragmatic trial (ISIS-2 1988) important features of which included:
- broad, simple inclusion criteria;
- central randomization;
- no requirement for additional biological samples or extraneous data collection; and
- use of the simple, unambiguous primary outcome of all-cause mortality.
Platform design
The trial platform design (Berry 2015) has meant that treatment arms could be dropped or added during the process of running RECOVERY trials. For example, on 23 May 2020, a convalescent plasma arm was added (using a factorial design) – a treatment first used more than 100 years ago (Luke 2006).
Because there were four arms in the initial RECOVERY trials, the control arm was made twice as large as the individual treatment arms. Since a 2:1 ratio has nearly the same power as a 1:1 ratio for the same total numbers, the 2:1 ratio of controls: treatment for each arm improved the power of each comparison (Park et al. 2020).
Rapid set up, recruitment, and reporting
RECOVERY has been remarkable, going from first meeting to first patient recruited in a record-setting nine days, recruiting 13% of all COVID-19 hospitalised patients in the UK during the first COVID-19 wave; and a few months later giving clear answers on the effectiveness of dexamethasone. Importantly, to minimise delay, results were reported in MedRxiv in June (RECOVERY 2020a MedRxiv), and a preliminary report was published in the New England Journal of Medicine the following month; but the full publication was not published until February 2021 (RECOVERY Collaborative Group N Engl J Med 2021a). At the same time, RECOVERY reported that no beneficial effect of hydroxychloroquine and lopinavir had been detected (RECOVERY Collaborative Group N Engl J Med 2020b; RECOVERY Collaborative Group Lancet 2020c). A year later RECOVERY reported beneficial effects of tocilizumab and Regeneron’s monoclonal antibody cocktail (RECOVERY Collaborative Group Lancet 2021b; RECOVERY Collaborative Group MedRxiv 2021c) and that no benefit had been detected from aspirin, colchicine, or convalescent plasma in hospitalized COVID-19 patients (RECOVERY Collaborative Group MedRxiv 2021d; RECOVERY Collaborative Group MedRxiv 2021e; RECOVERY Collaborative Group Lancet 2021f).
Some keys to the successful recruitment by RECOVERY trials were:
- simple, pragmatic designs;
- the pre-existing National Health Service clinical trials network; and
- strong national support from the Chief Medical Officers in England, Scotland, Wales and Northern Ireland.
The pre-existing National Institute for Health Research Network in the UK meant that there was a decision-communication structure to prioritise the RECOVERY trial platform, and research nurses in place at recruiting centres. Because of its simple features, many of the less research-experienced hospitals became among the best recruiters to RECOVERY trials.
The success of the RECOVERY platform trials has been facilitated by the willingness of UK National Health Service doctors to randomize unproven treatments (rather than, as in many other countries, obstructing randomization by incorporating unproven drugs into local practice guidelines).
A strong letter of support from UK Chief Medical Officers emphasized that support for RECOVERY was a part of clinical care: “Use of treatments outside of a trial, where participation was possible, is a wasted opportunity to create information that will benefit others” (https://www.recoverytrial.net/files/professional-downloads/the-importance-of-covid-19-clinical-trials.pdf )
CONCLUSIONS
The features above are not unique to RECOVERY but the combination of all of them appears to have been ground-breaking. While there have been several previous platform trials (Park 2019), most have been in oncology. One previous pandemic attempt occurred in the 2014 Ebola outbreak in West Africa, but it was not possible to launch it before the outbreak had subsided (Park 2019).
Although the evidence generated using the RECOVERY trials platform is clinically important, the lessons for the medical and research community about processes are perhaps of even greater importance and worthy of detailed study. Simple, large-scale trials are key to obtaining clear clinical answers to vital questions. The RECOVERY trial platform has demonstrated that it is possible to answer multiple treatment questions reliably during a pandemic. This is an important lesson for future pandemics, but it is also relevant to non-pandemic treatment questions(Collins 2020). Funders and trialists would do well to study carefully what made the success of RECOVERY possible, and what other lessons there are for adapting the clinical trials ecosystem during pandemics (Clinical Trials Transformation Initiative).
Note: This James Lind Library article will be updated at intervals to add information about the development of the RECOVERY trial platform and the treatment evidence obtained.
Acknowledgements
We would like to thank Peter Horby and Martin Landray for helpful discussions in developing this report.
This James Lind Library article has been republished in the Journal of the Royal Society of Medicine 2021;114:443-446. Print PDF
References
Berry SM, Connor JT, Lewis RJ (2015). The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 313:1619–1620.
Clinical Trials Transformation Initiative: Clinical Trials Issues Related to COVID-19.
Collins R, Bowman L, Landray M, Peto R (2020). The magic of randomization versus the myth of real-world evidence. New England Journal of Medicine 382:674–678.
Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) (1986). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1: 397-402.
ISIS-2 (second International Study of Infarct Survival) Collaborative Group (1988).
Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2:349–360.
Luke TC, Kilbane EM, Jackson JL, Hoffman SL (2006). Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Medicine 145:599-609. doi: 10.7326/0003-4819-145-8-200610170-00139. Epub 2006 Aug 29. PMID: 16940336.
Maggioni AP, Franzosi MG, Fresco C, Turazza F, Tognoni G (1990). GISSI trials in acute myocardial infarction. Rationale, design, and results. Chest 97(4 Suppl):146S-150S.
Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ (2020). An overview of platform trials with a checklist for clinical readers. Journal of Clinical Epidemiology 125:1-8.
Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, Lester RT, Thorlund K, Mills EJ (2019). Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 20:572.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1976). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. British Journal of Cancer 34:585-6.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. British Journal of Cancer 35:1-39.
Peto R (1978). Clinical trial methodology. Biomedicine 28 (special issue):24-36.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2020a). Dexamethasone in hospitalized patients with Covid-19 – Preliminary Report. medRxiv, 22 Jun 2020. DOI: 10.1101/2020.06.22.20137273
RECOVERY Collaborative Group (2020b). Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 383:2030-2040. doi: 10.1056/NEJMoa2022926. Epub 2020 Oct 8.
RECOVERY Collaborative Group (2020c). Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 396:1345-1352. doi: 10.1016/S0140-6736(20)32013-4. Online ahead of print.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2021a). Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 384:693-704. doi: 10.1056/NEJMoa2021436. Epub 2020 Jul 17. PMID: 32678530; PMCID: PMC7383595.
RECOVERY Collaborative Group (2021b). Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0.
RECOVERY Collaborative Group (2021c). Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1
RECOVERY Collaborative Group (2021d). Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.06.08.21258132v1
RECOVERY Collaborative Group (2021e). Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.05.18.21257267v1
RECOVERY Collaborative Group (2021f). Cownvalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021 397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7. Epub 2021 May 14.
Tikkinen KAO, Malekzadeh R, Schlegel M, Rutanen J, Glasziou P (2020). COVID-19 clinical trials: learning from exceptions in the research chaos. Nature Medicine 26:1671-1672. doi: 10.1038/s41591-020-1077-z.
Tognoni G, Franzosi MG, Garattini S (2018). Embedding patient- and public health-oriented research in a national health service: the GISSI experience. JLL Bulletin: Commentaries on the history of treatment evaluation. https://www.jameslindlibrary.org/articles/embedding-patient-and-public-health-oriented-research-in-a-national-health-service-the-gissi-experience/
WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S (2021). Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. PMID: 33264556; PMCID: PMC7727327.
Yusuf S, Collins R, Peto R (1984). Why do we need some large, simple randomized trials? Statistics in Medicine 3:409–422.
Glasziou PP, Tikkinen KAO (2021). The RECOVERY trial platform: a milestone in the development and execution of treatment evaluation during an epidemic.
© Paul Glasziou, Centre for Research in Evidence-Based Practice, Faculty of Health Sciences and Medicine, Bond University, Gold Coast, Queensland 4229, Australia. Email: pglaszio@bond.ac.uk
Cite as: Glasziou PP, Tikkinen KAO (2021). The RECOVERY trial platform: a milestone in the development and execution of treatment evaluation during an epidemic. JLL Bulletin: Commentaries on the history of treatment evaluation (https://www.jameslindlibrary.org/articles/the-recovery-trial-platform-a-milestone-in-the-development-and-execution-of-treatment-evaluation-during-an-epidemic/)
Creation of the RECOVERY trial platform during the COVID-19 pandemic has been a milestone in clinical trial processes. The RECOVERY trial platform has been built on Richard Peto’s promotion since the 1970’s of large, simple trials using factorial designs to make more than two treatment comparisons (Peto et al. 1976; 1977; Peto 1978; Yusuf et al. 1984). The RECOVERY trial platform (hereafter ‘RECOVERY’) has extended that model with some novel design features, particularly a very rapid set up and recruitment process. RECOVERY has set new benchmarks for trial efficiency. Importantly, results obtained through RECOVERY have had a strong impact on clinical practice and clinical guidelines during the COVID-19 pandemic – a feat not achieved during any previous pandemic.
In parallel to RECOVERY, WHO has also created and coordinated a large multi-country trial platform with a similar protocol to RECOVERY. By the end of January 2021, WHO’s SOLIDARITY platform had enrolled over 14,000 patients in more than 400 hospitals in over 30 countries, and it has provided by far the most statistically precise estimate of the effects of remdesivir in COVID-19. Interim remdesivir results were published in December 2020 (WHO Solidarity Trial Consortium 2020) and the final analyses are expected to be published in summer 2021. At the end of January 2021, trial recruitment using the SOLIDARITY platform was paused to deal with several logistic difficulties, but recruitment is expected to resume during July 2021.
Although the important lessons resulting from the RECOVERY and SOLIDARITY trial platform experiences are similar (Tikkinen et al. 2020), in this commentary we focus on RECOVERY. In brief, the ‘Randomised Evaluation of COVID-19 therapy (RECOVERY)’ trial is a randomized, controlled, open-label, adaptive, trial platform enabling comparisons of a range of possible treatments with usual care in patients hospitalized with COVID-19. The initial four experimental arms were: (i) lopinavir-ritonavir (commonly used to treat HIV), (ii) low-dose dexamethasone, (iii) azithromycin (a commonly used antibiotic), and (iv) hydroxychloroquine. Using its adaptive design and successful recruitment, RECOVERY has also already assessed the effects of (v) aspirin, (vi) colchicine, (vii) convalescent plasma, (viii) Regeneron’s monoclonal antibody cocktail, and (ix) tocilizumab. As of 15 July 2021, RECOVERY is being used to test (x) baricitinib (an immunomodulatory drug used in rheumatoid arthritis), (xi) dimethyl fumarate (an immunomodulatory drug used in psoriasis and multiple sclerosis), and (xii) high-dose vs standard dose of corticosteroids.
The RECOVERY protocol is available for use by investigators everywhere to design their own randomized trials to help identify treatments for COVID-19 with important beneficial effects. https://www.isrctn.com/ISRCTN50189673
Some special features of RECOVERY include:
Simplicity
The protocol, written over two days (see Timeline box) was adapted from the large, simple, pragmatic trials exemplified by the ISIS trials (e.g., ISIS-2 1988) and the GISSI trials (e.g., GISSI 1986; Maggioni et al. 1990; Tognoni et al 2018) using broad, simple, inclusion criteria and minimising data collection to enable participation by COVID-19-stretched hospitals. The design of RECOVERY was based on ISIS-2 protocol – a large, simple, pragmatic trial (ISIS-2 1988) important features of which included:
Platform design
The trial platform design (Berry 2015) has meant that treatment arms could be dropped or added during the process of running RECOVERY trials. For example, on 23 May 2020, a convalescent plasma arm was added (using a factorial design) – a treatment first used more than 100 years ago (Luke 2006).
Because there were four arms in the initial RECOVERY trials, the control arm was made twice as large as the individual treatment arms. Since a 2:1 ratio has nearly the same power as a 1:1 ratio for the same total numbers, the 2:1 ratio of controls: treatment for each arm improved the power of each comparison (Park et al. 2020).
Rapid set up, recruitment, and reporting
RECOVERY has been remarkable, going from first meeting to first patient recruited in a record-setting nine days, recruiting 13% of all COVID-19 hospitalised patients in the UK during the first COVID-19 wave; and a few months later giving clear answers on the effectiveness of dexamethasone. Importantly, to minimise delay, results were reported in MedRxiv in June (RECOVERY 2020a MedRxiv), and a preliminary report was published in the New England Journal of Medicine the following month; but the full publication was not published until February 2021 (RECOVERY Collaborative Group N Engl J Med 2021a). At the same time, RECOVERY reported that no beneficial effect of hydroxychloroquine and lopinavir had been detected (RECOVERY Collaborative Group N Engl J Med 2020b; RECOVERY Collaborative Group Lancet 2020c). A year later RECOVERY reported beneficial effects of tocilizumab and Regeneron’s monoclonal antibody cocktail (RECOVERY Collaborative Group Lancet 2021b; RECOVERY Collaborative Group MedRxiv 2021c) and that no benefit had been detected from aspirin, colchicine, or convalescent plasma in hospitalized COVID-19 patients (RECOVERY Collaborative Group MedRxiv 2021d; RECOVERY Collaborative Group MedRxiv 2021e; RECOVERY Collaborative Group Lancet 2021f).
Some keys to the successful recruitment by RECOVERY trials were:
The pre-existing National Institute for Health Research Network in the UK meant that there was a decision-communication structure to prioritise the RECOVERY trial platform, and research nurses in place at recruiting centres. Because of its simple features, many of the less research-experienced hospitals became among the best recruiters to RECOVERY trials.
The success of the RECOVERY platform trials has been facilitated by the willingness of UK National Health Service doctors to randomize unproven treatments (rather than, as in many other countries, obstructing randomization by incorporating unproven drugs into local practice guidelines).
A strong letter of support from UK Chief Medical Officers emphasized that support for RECOVERY was a part of clinical care: “Use of treatments outside of a trial, where participation was possible, is a wasted opportunity to create information that will benefit others” (https://www.recoverytrial.net/files/professional-downloads/the-importance-of-covid-19-clinical-trials.pdf )
CONCLUSIONS
The features above are not unique to RECOVERY but the combination of all of them appears to have been ground-breaking. While there have been several previous platform trials (Park 2019), most have been in oncology. One previous pandemic attempt occurred in the 2014 Ebola outbreak in West Africa, but it was not possible to launch it before the outbreak had subsided (Park 2019).
Although the evidence generated using the RECOVERY trials platform is clinically important, the lessons for the medical and research community about processes are perhaps of even greater importance and worthy of detailed study. Simple, large-scale trials are key to obtaining clear clinical answers to vital questions. The RECOVERY trial platform has demonstrated that it is possible to answer multiple treatment questions reliably during a pandemic. This is an important lesson for future pandemics, but it is also relevant to non-pandemic treatment questions(Collins 2020). Funders and trialists would do well to study carefully what made the success of RECOVERY possible, and what other lessons there are for adapting the clinical trials ecosystem during pandemics (Clinical Trials Transformation Initiative).
Note: This James Lind Library article will be updated at intervals to add information about the development of the RECOVERY trial platform and the treatment evidence obtained.
Acknowledgements
We would like to thank Peter Horby and Martin Landray for helpful discussions in developing this report.
This James Lind Library article has been republished in the Journal of the Royal Society of Medicine 2021;114:443-446. Print PDF
References
Berry SM, Connor JT, Lewis RJ (2015). The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 313:1619–1620.
Clinical Trials Transformation Initiative: Clinical Trials Issues Related to COVID-19.
Collins R, Bowman L, Landray M, Peto R (2020). The magic of randomization versus the myth of real-world evidence. New England Journal of Medicine 382:674–678.
Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) (1986). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1: 397-402.
ISIS-2 (second International Study of Infarct Survival) Collaborative Group (1988).
Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2:349–360.
Luke TC, Kilbane EM, Jackson JL, Hoffman SL (2006). Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Medicine 145:599-609. doi: 10.7326/0003-4819-145-8-200610170-00139. Epub 2006 Aug 29. PMID: 16940336.
Maggioni AP, Franzosi MG, Fresco C, Turazza F, Tognoni G (1990). GISSI trials in acute myocardial infarction. Rationale, design, and results. Chest 97(4 Suppl):146S-150S.
Park JJH, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ (2020). An overview of platform trials with a checklist for clinical readers. Journal of Clinical Epidemiology 125:1-8.
Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, Lester RT, Thorlund K, Mills EJ (2019). Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 20:572.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1976). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. British Journal of Cancer 34:585-6.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. British Journal of Cancer 35:1-39.
Peto R (1978). Clinical trial methodology. Biomedicine 28 (special issue):24-36.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2020a). Dexamethasone in hospitalized patients with Covid-19 – Preliminary Report. medRxiv, 22 Jun 2020. DOI: 10.1101/2020.06.22.20137273
RECOVERY Collaborative Group (2020b). Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 383:2030-2040. doi: 10.1056/NEJMoa2022926. Epub 2020 Oct 8.
RECOVERY Collaborative Group (2020c). Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 396:1345-1352. doi: 10.1016/S0140-6736(20)32013-4. Online ahead of print.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2021a). Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 384:693-704. doi: 10.1056/NEJMoa2021436. Epub 2020 Jul 17. PMID: 32678530; PMCID: PMC7383595.
RECOVERY Collaborative Group (2021b). Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397:1637-1645. doi: 10.1016/S0140-6736(21)00676-0.
RECOVERY Collaborative Group (2021c). Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1
RECOVERY Collaborative Group (2021d). Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.06.08.21258132v1
RECOVERY Collaborative Group (2021e). Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv 2021, available at https://www.medrxiv.org/content/10.1101/2021.05.18.21257267v1
RECOVERY Collaborative Group (2021f). Cownvalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021 397:2049-2059. doi: 10.1016/S0140-6736(21)00897-7. Epub 2021 May 14.
Tikkinen KAO, Malekzadeh R, Schlegel M, Rutanen J, Glasziou P (2020). COVID-19 clinical trials: learning from exceptions in the research chaos. Nature Medicine 26:1671-1672. doi: 10.1038/s41591-020-1077-z.
Tognoni G, Franzosi MG, Garattini S (2018). Embedding patient- and public health-oriented research in a national health service: the GISSI experience. JLL Bulletin: Commentaries on the history of treatment evaluation. https://www.jameslindlibrary.org/articles/embedding-patient-and-public-health-oriented-research-in-a-national-health-service-the-gissi-experience/
WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S (2021). Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497-511. doi: 10.1056/NEJMoa2023184. Epub 2020 Dec 2. PMID: 33264556; PMCID: PMC7727327.
Yusuf S, Collins R, Peto R (1984). Why do we need some large, simple randomized trials? Statistics in Medicine 3:409–422.