Paul Arnor Owren (1905–1990)
In 1943, during the German occupation of Norway, a 29-year-old woman was admitted to Medical Department A at The National Hospital (Rikshospitalet) in Oslo. She would become crucial to a tradition of coagulation research in Norway after World War II. The patient had had prolonged nosebleeds, severe menorrhagia (menstrual bleeding), and other signs of a bleeding disorder. As the patient was a woman, classical hemophilia was out of the question so a search for the reason for her symptoms began (Stormorken 2003).
The patient’s physician was Paul Arnor Owren, a young doctor who had graduated from Medical School at The University of Oslo in 1931. After some years working as a general practitioner in Lillehammer he left his practice and became a fox farmer, to provide fox pelts for the clothing industry (!). Owren even published a few papers on insemination in foxes. However, in 1939 he returned to medicine and The National Hospital (Stormorken 2000) (Figure 1).

Figure 1. Professor Helge Stormorken (1922–2019) published a biography of Paul Owren in 2000 (Stormorken 2000).
Owren found the woman with bleeding of particular interest. With only scarce resources, he began to investigate her coagulation system. The University of Oslo had been closed by the German occupiers, so the prerequisites for research were limited. Furthermore, Owren had no previous academic training. Despite these obstacles, based on investigating his patient, Owren succeeded in identifying a new clotting factor. He named it Factor V because, since 1905, Paul Morawitz (1879–1936) had already identified four clotting factors (Bächli 2000).
In 1944, Owren presented his findings at a meeting of The Norwegian Academy of Science and Letters, and in 1946, at the XVII International Congress of Physiology in Oxford. An account of his research was published in The Lancet in 1947 (Owren 1947a), and he defended a doctoral thesis based on his research later that year (Owren 1947b).
Paul Owren became head of the Medical Department A at The National Hospital and full professor at the University of Oslo in 1949 – a position he held for the following 20 years. He continued his research and delivered important contributions to understanding the mechanisms of coagulation. He was the first to publish a case series of long-term dicoumarol therapy in Norway (Owren 1953). He also developed a method for monitoring anticoagulation therapy (“Thrombotest”) that made him both famous and wealthy. He became a world-renowned researcher on coagulation and established his own research group and later an Institute for Thrombosis Research. This attracted scores of talented researchers: a generation of Norwegian internists and hematologists obtained their scientific training at “Stall Owren”.
Christopher Juel Bjerkelund (1916–2002)
One of the many young doctors whose medical careers began under Owren’s leadership was Christopher Juel Bjerkelund (Figure 2). Bjerkelund graduated from the Medical School at the University of Oslo in 1945 and he held short hospital posts in Trondheim and Kristiansand before beginning his training as an internist at Medical Department A at The National Hospital in 1947. In the preface of his thesis ten years later he wrote: ”The basic knowledge of blood coagulation and anticoagulant therapy which I was taught as an assistant and research fellow in [Owren’s] department and laboratory was a necessary prerequisite for this investigation” (Bjerkelund 1957)

Figure 2. Christopher Bjerkelund married Agnes (née Riiber), a nurse, in 1944. They had one child, Carl Eivind (b. 1949). Photo: Private, reproduced with permission from Agnes and Carl Eivind Bjerkelund.
In 1950, Bjerkelund moved to Department VIII at Ullevål Hospital, a municipal hospital in Oslo, and trained there as a cardiologist under Professor Carl Müller (1886–1983), well known because of his research on familial hypercholesterolaemia (Müller-Harbitz’ disease) collaborating with the Norwegian pathologist Francis Harbitz (1867–1950) (Müller 1938). Bjerkelund remained at this department for 13 years and completed his doctoral thesis there in 1957 (Bjerkelund 1957). In 1963 he returned to The National Hospital as an assistant professor and consultant until 1969, when he became full professor and head of internal medicine at Aker Hospital, another municipal hospital in Oslo. He retired in 1986 and practiced privately in Oslo for many years.
Bjerkelund became a prominent figure in Norwegian cardiology in the 1950s. He was chair of the Norwegian Society of Cardiology and the Norwegian Society for Internal Medicine, and a Fellow of the American College of Cardiology. He was a member of the editorial board of The Journal of the Norwegian Medical Association from 1976 to 1988. As a former editor I remember him as a kind man, who could use a firm tone of voice when needed. His only child, Carl Eivind Bjerkelund (b. 1949), an anesthesiologist, describes his father as a decisive man who did not hesitate to participate in academic debates (CE Bjerkelund, personal communication, 15 May 2023). When, in the 1960s, Owren advocated the use of linolenic acid for the prevention of myocardial infarction (Owren et al. 1964), Bjerkelund was among the many who opposed his former boss, a position that was later confirmed in clinical studies (Natvig et al. 1968). Next to medicine, music, literature and the visual arts were Christopher Bjerkelund’s most important interests. He died in 2002 at the age of 86. At the time of writing this article (2023), Christopher Bjerkelund’s widow, Agnes Bjerkelund (b. 1920), is living alone in her own house at the age of 102.
Bjerkelund’s research on myocardial infarction
Before World War II, the incidence of myocardial infarction was increasing slightly in Norway but fell during the war (Strøm & Jensen 1951). Probably due to altered nutrition and readier access to tobacco there was a dramatic increase in incidence after the war, especially among middle-aged men. Among men in Oslo aged 40–59 years old there was a fourfold increase in the incidence of myocardial infarction between 1945 and 1950, from 4/10 000 per year to 16/10 000 (Life Insurance Companies’ Institute for Medical Statistics at the Oslo City Hospitals 1956).
Anticoagulant therapy for thromboembolic diseases had been introduced in the 1940s and vitamin-K antagonists were used for long-term treatment (Franchini et al. 2016). Dicoumarol, a drug with bioactive properties, was initially discovered during investigation of a mysterious disease of cattle. The drug was developed as a pharmaceutical product and replaced by warfarin in the 1950s (Timson et al. 2017). Due to the war, dicoumarol was not available in Norway until 1947 and warfarin was not approved by the Norwegian drug authorities until 1962.
Of Christopher Bjerkelund’s 53 entries in PubMed, a third are on myocardial infarction and/or anticoagulation. As a sign of the times in medical publishing, 32 of his papers were in Norwegian and only 21 in English. Bjerkelund had a special interest in the administration of dicoumarol and published a six-page article on this in The Lancet in 1953 (Bjerkelund 1953). His study on long term treatment with dicoumarol to prevent recurrences after myocardial infarction (Bjerkelund 1957) established a path for later Norwegian studies on this topic.
The title of Bjerkelund’s publication suggests that it was the first Norwegian thesis at the University of Oslo to be labelled a “controlled clinical trial” (Larsen 2014). It was published as a 212-page monograph and as a supplement to Acta Medica Scandinavica (from 1989, Journal of Internal Medicine) (Bjerkelund 1957)
Bjerkelund defended his thesis on 19 October 1957. His opponents were Paul Owren (Bjerkelund’s former boss) and Hans Jacob Ustvedt (1903–1982), who five years later left medicine to become head of the Norwegian Broadcasting Corporation.
Bjerkelund’s assessment of anticoagulant prophylaxis after myocardial infarction.
The increasing incidence of myocardial infarction after World War II led to an intensive search for treatment and preventive measures. Reports were published in 1948 on the benefit of anticoagulation with dicoumarol in the acute phase of myocardial infarction (Wright et al. 1948). Whether such treatment also had a prophylactic effect on recurrent heart attacks was unclear.
In a literature review in his thesis, Bjerkelund concluded that there were “no convincing statistical results available from carefully planned and controlled therapeutic trials”. “[A]rranging the best possible controlled clinical trial in which the course of the disease can be compared statistically in treated and untreated patients from the same period and the same source… has been the object and is the justification of this study” (Bjerkelund 1957, p 42).
Bjerkelund’s research question was: “Will continuous anticoagulant administration after acute myocardial infarction improve the prognosis in a given patient in relation to a similar patient without this form of treatment?” (Bjerkelund 1957, p 15).
Reduction in mortality, incidence of recurrent infarction and “perhaps a decrease in the number of thromboembolic complications” were his chosen endpoints.
Study design
As far as I am aware, Bjerkelund’s study was the first controlled clinical intervention study in Norway. Despite its possible biases and methodological flaws, it was an important precursor to later randomized controlled trials (RCTs).
Bjerkelund stated that the “factors affecting the prognosis should be as evenly distributed as possible between the two groups to be compared” (Bjerkelund 1957, p 43), and he discussed different ways of achieving this:
- Stratification, where all the patients in the same prognostic stratum would “for example by drawing lots, be distributed between the treated and control groups”.
- A chance distribution without stratification “for example, the patients can be allotted to the two groups alternately in the order they are admitted to hospital. Or, they could be allotted according to whether they were admitted on even or odd dates, or on the basis of the date of birth being even or odd”.
In the event he rejected both these methods because “it was obvious that one of the greatest difficulties (…) would be to keep the control group intact” (Bjerkelund 1957, p 44). The problem of drug sharing and patient preferences in randomized trials remains recognized as an issue today (Corbett et al. 2016; Moodley et al. 2016).
Bjerkelund had realized by 1957 that “Any new treatment is, in the eyes of the public, always the best, and there was reason to fear that, as the patients in the control group gradually came to know that some patients were treated in this way [with anticoagulation], they would want the same for themselves” (Bjerkelund 1957, p 44). Patients at that time were housed in wards with up to 22 beds and it was unavoidable that they would – during a month’s stay (which was common) – discuss their treatment and follow up. Bjerkelund feared that patients allocated to the control group would demand anticoagulation when they heard of the possibility. Therefore, he decided “on careful consideration” that it would be “best to let the question of to which group a patient was to be included would depend on to which department he had been admitted” (Bjerkelund 1957, p 45).
Ullevål Hospital had three departments of internal medicine (VII, VIII, IX). Admissions were handled by a hospital bed service outside the hospital, the choice of department being based on the number of empty beds. The treatment procedure for myocardial infarction was identical in the three departments, with anticoagulation prophylaxis during the first month after an acute infarction. In department VII anticoagulation would be terminated after one month and patients assigned to the control group. In department VIII long term anticoagulation therapy would continue with patients assigned to the intervention group. In department IX (the smallest of the three) patients would be assigned alternately either to the control group or to the intervention group for periods of about half a year.
Bjerkelund worked alone and was in personal charge of the follow-up of all patients included in the trial.
Patients in the intervention group had to attend follow-up assessments at intervals of a few weeks for their coagulation status to be monitored. Bjerkelund decided that follow-up and blood tests of those receiving placebo was impracticable and unethical, so they were assessed every 3–4 months.
In the Preface to his thesis Bjerkelund acknowledges Erling Sverdrup (1917–1994), a pioneer in Norwegian mathematical statistics, for “invaluable help and advice in the statistical investigation of my observations” (Bjerkelund 1957, p 5). No statistical power calculation was done to estimate the number of participants needed, but that would have been rare in the 1950s (Descôteaux 2007; Cohen 1962).
The study population
Patients under 76 years who had survived an acute myocardial infarction for at least one month were included. Six exclusion criteria were defined, before inclusion, for example, mental illness and contraindications to the use of anticoagulants.
The study population consisted of 277 patients with myocardial infarction. They were included from July 1950 to July 1953 and followed up to February 1956. 138 patients were assigned to the intervention group and 139 to the control group. The two groups were found to be similar regarding age, gender, social standing, and clinical status. Bjerkelund concluded that “the statistical comparison of the patients in the treated and control groups provides a good basis for stating that the patients were allotted by chance to the two groups” (Bjerkelund’s emphasis) (Bjerkelund 1957, p 87).
According to predetermined criteria, 12 patients were excluded from the intervention group and 14 from the control group before observation. I have constructed a flow chart of the study using Bjerkelund’s data (Figure 3).
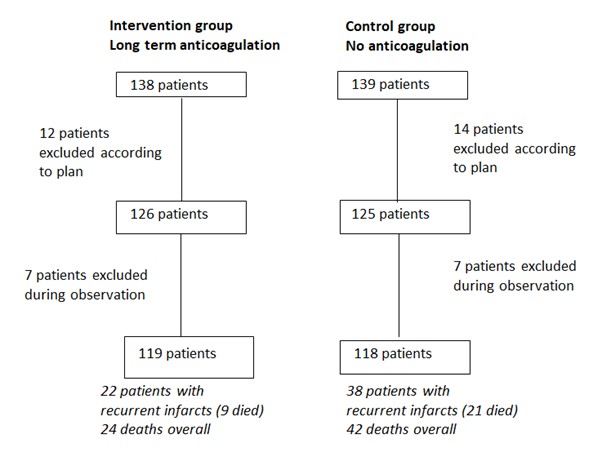
Figure 3. Flow chart of the two groups recruited between July 1950 and July 1953 and observed until February 1956 produced by Magne Nylenna based on (Bjerkelund 1957).
Findings
During the observation period, a further 14 patients were excluded, 7 from each group. The reasons for dropout in the intervention group were 2 patients with serious bleeds, 1 with progressive dementia, and 4 who refused further participation. In the control group, 6 patients received long-term anticoagulation from other doctors, or for other reasons, and 1 refused to participate. Patients with short term anticoagulation during the observation period in the control group, or intermittent irregularities or interruptions (for example, for surgery) in anticoagulation in the intervention group, were included in the final analyses. Recurrences of myocardial infarction and mortality were analyzed according to timing of incidents and age of participants.
By February 1956, both the incidence of recurrences and the mortality were lower in the intervention group than in the control group. Bjerkelund reported statistically significant effects of anticoagulation were found among patients under 60 years of age during the first 12 months of treatment. Twenty-two patients in the intervention group had recurrent infarctions and 9 of them died, compared to 38 patients in the control group, of whom 21 died. A total of 24 deaths were registered in the intervention group (23 from cardiovascular disease) compared to 42 deaths in the control group (38 from vascular disease). Bjerkelund emphasizes that this does not mean “that mortality in the treated group is only 4/7 as large in the control group” (Bjerkelund 1957, p 140), but it is hard to understand the details of his statistical analyses and his use of expressions such as “force of recurrences” and “force of mortality”. Luckily this is not crucial to the importance of the study design.
A reanalysis of the raw data yields a statistically significant effect of anticoagulation on recurrences and mortality (Table 1).
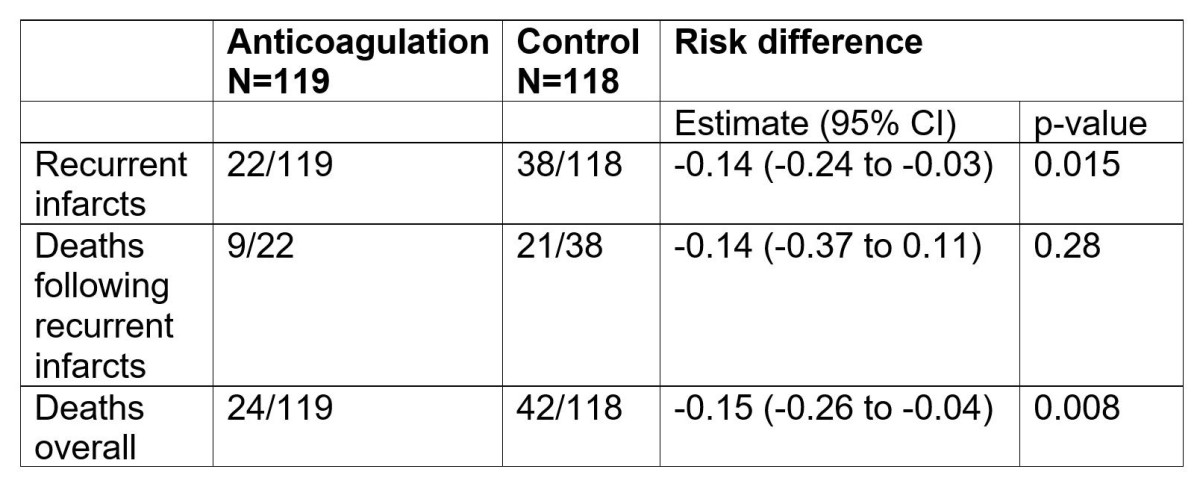
Table 1. Reanalyses of raw data from Bjerkelund’s thesis (Bjerkelund 1957). 95% Confidence Intervals (CI) calculated using the Newcombe Hybrid score method (Fagerland et al. 2015). P-value calculated using the Pearson chi squared test.
Comments
Research method
Christopher Bjerkelund’s paper is first and foremost important because of the date (1957) it was published. Even with its shortcomings it is sophisticated for its time.
Bjerkelund was aware of the need for randomization in controlled clinical trials and of different ways of achieving this. His reason for leaving the responsibility for allotting patients to the two comparison groups based on hospital department admission to an external bed bureau instead of drawing lots was primarily to reduce the risk of losing “untreated” patients. After “careful consideration” he decided that the risk of many control patients demanding anticoagulation was more important than the disadvantages of suboptimal allocation. Despite these precautions 6 patients in the control group were given long-term anticoagulation and excluded from the final analyses.
This exclusion, as well as the exclusion of 7 dropouts from the intervention group, is a breach of the intention-to-treat principle as defined today. The true effect of an intervention, like anticoagulation in Bjerkelund’s study, is influenced by more than the biological effect of dicoumarol, for example, by variations in compliance, adherence to a treatment plan, and by other factors. Accordingly, all patients intended for treatment or control should be included in the final analysis (Detry and Lewis 2014; Montori and Guyatt 2001; Chalmers et al. 2023). The 7 dropouts in the intervention group should have been included in an intention-to-treat analysis. It is less clear how data relating to the 6 patients in the control group who were prescribed long-term anticoagulation should be handled. Bjerkelund specified that patients in the intervention group who had had periods without anticoagulation and patients in the control group who had periods with anticoagulation were to be included in the analysis. For this reason his study design should be characterized as a ‘modified intention-to-treat analysis’ (Detry et al. 2014; Chalmers et al. 2023).
Bjerkelund’s “careful consideration” does not meet today’s requirements for a randomized controlled trial, even though he discussed the limitations of his research openly. By mid-20th century there was more room for personal appraisal in all fields of medicine. “Clinical freedom” had not yet been replaced by procedures, guidelines and evidence-based medicine. Clinical research was not regulated by international conventions, ethics committees and requirements for informed consent.
By then the quality of research methods had already become a topic of heated discussions. One of my first informants for this paper was Christian Borchgrevink (b.1924) who worked with Owren in the late 1950s and early 1960s and knew Bjerkelund and his work well. When I erroneously referred to Bjerkelund’s thesis as a randomized trial Borchgrevink corrected me immediately, urging me to “remember, it was not a randomized trial. He [Bjerkelund] allocated patients by department, not by chance” (CF Borchgrevink, personal communication, 16 May 2023). In 1966, Peter Armitage and Christian Borchgrevink (Armitage and Borchgrevink 1966) strongly criticized a study reported two years earlier on the prevention of recurrences of myocardial infarction. Loyal L. Conrad et al. had written that past trials “have been designed so poorly that they do not qualify as controlled medical trials” (Conrad et al. 1964). Armitage and Borchgrevink in return accused Conrad et al. for “inappropriate statistical methods” and “inadequate number of patients” (Armitage and Borchgrevink 1966).
The first textbook on controlled trials in Norway was published in 1966 by Erik Enger (1927–2016). He stated that “Previous studies of anticoagulant therapy in this country have avoided placebo and the double-blind technique because of practical, ethical and medico-legal difficulties” (Enger 1966 p 99) (translated by MN). Forty years later, Bjerkelund’s trial and studies from the 1960s were commented as follows: “Based on today’s standards, many will probably claim that these studies were too small and with too short a follow-up to be able to demonstrate with certainty an effect on so-called “hard endpoints.” (Otterstad and Brosstad 2007) (translated by MN).
In an early example of a systematic review on anticoagulation in myocardial infarction, Thomas Chalmers (1917–1995) and his colleagues pointed out “the poor quality of the papers from the nonrandom trials, which revealed the largest anticoagulant effect on case fatality rates, and the minimal differences between treatments in most of the random control trials” (Chalmers et al. 1977).
Christopher Bjerkelund (Bjerkelund 1957) and Christian Borchgrevink (Borchgrevink 1960) paved the way for a series of later Norwegian RCTs in preventive cardiology conducted according to today’s strict requirements. Terje Pedersen (b. 1945) was principal investigator for the studies of timolol (Pedersen 1985) and simvastatin (Pedersen et al. 1994). Harald Arnesen (b.1938) led the two Norwegian Warfarin Re-Infarction Studies (WARIS) published in 1990 and 2002 (Smith et al. 1990; Hurlen et al. 2002). Kaare H. Bønaa (b. 1952) was principal investigator in the NORVIT-trial assessing the effect of lowering homocysteine (Bønaa et al. 2006).
Anticoagulation
Bjerkelund’s findings in 1957 were in line with other contemporary studies. In an analysis of nine controlled trials of long-term anticoagulation after myocardial infarction of which Bjerkelund’s was the first, mortality was 20 % lower in men in the intervention group than in controls (International Anticoagulant Review Group 1970). The review group could not, however, reach agreement on clinical recommendations which, in a review in 1981 (also including Bjerkelund’s study) (Loeliger 1981), was seen as the main reason for abandoning routine oral anticoagulation. The use of oral anticoagulants remained controversial during the last two decades of the 20th century despite studies that showed a beneficial effect (Smith et al. 1990; Anand and Yusuf 1999; Hurlen et al. 2002). The risk of bleeding complications was one of the main objections.
Direct oral anticoagulants (DOACs), replacing traditional vitamin K antagonists, have improved the safety of anticoagulation significantly (Connors 2018), but other long-term treatments have limited the indication for anticoagulant drugs in coronary heart disease.
By the turn of the century antiplatelet therapy had become a game changer (Hirsch 2008). Beneficial effects of acetylsalicylic acid (aspirin) in myocardial infarction had already been documented in 1988 (ISIS-2 1988) and suggested a survival advantage of up to ten years (Baigent et al. 1998). Adding oral anticoagulation to aspirin seemed to have little additional effect (OASIS 2001). The introduction of new antiplatelet agents such as clopidogrel (CURE 2001) established an undisputed requirement of dual antiplatelet therapy (aspirin + a P2Y12 inhibitor). A combination of one particular DOAC (rivaroxaban) and aspirin (Eikelboom et al. 2017) was termed a “dual-pathway” alternative (Atar et al. 2014) and showed clinical efficacy.
According to the pertinent European Society of Cardiology Guidelines (2018; 2021), antiplatelet therapy is now the first choice for preventing recurrences after myocardial infarction. The extensive use of percutaneous coronary intervention (PCI) and intracoronary stent implantation in acute coronary syndromes demands effective antiplatelet therapy to prevent stent thrombosis. This explains to a great extent the shift from anticoagulation to antiplatelet therapy following myocardial infarction. The patients included in Bjerkelund’s study differ from post-infarction patients today with coronary artery stents and reduced infarct size. A remaining indication for anticoagulation is left ventricular thrombus following a large infarction. However, contemporary treatment includes effective reperfusion therapy in the acute phase to reduce infarct size and thus the risk of thrombus formation in the ventricle.
Today the main indications for oral anticoagulation are atrial fibrillation/ prevention of stroke, venous thromboembolism, pulmonary embolism, and status after heart valve replacement (Altiok and Marx 2018; Wadsworth et al. 2021).
Conclusion
Christopher Bjerkelund was undoubtedly ahead of his time, both in clinical research and in the use of anticoagulation. According to his obituary (Abildgaard and Orning 2002), just before he died, Bjerkelund saw the beneficial effects of long-term anticoagulation after myocardial infarction confirmed in the Norwegian WARIS II-study as a feather in his cap (Hurlen et al. 2002).
Acknowledgements
I am grateful to Stian Lydersen, Professor of Medical Statistics at the Norwegian University of Science and Technology, for his contribution to statistical analyses. I am also grateful to Professor Erlend Hem for help with literature searches, and to Professor Dan Atar, Professor Chr. Borchgrevink, Professor Dag Thelle, Professor Rune Wiseth, Dr Carl Eivind Bjerkelund and Dr Iain Chalmers for valuable contributions.
This James Lind Library article has been republished in the Journal of the Royal Society of Medicine 2024;117:77-84. Print PDF
References
Abildgaard U, Orning O (2002). Christopher Juel Bjerkelund. Aftenposten 16 July 2002.
Altiok E, Marx N (2018). Oral Anticoagulation Dtsch Arztebl Int 115: 776–783.
Anand SS, Yusuf S (1999). Oral anticoagulant therapy in patients with coronary artery disease: A meta-analysis. JAMA 282: 2058–2067.
Armitage P, Borchgrevink CF (1966). Prevention of recurrences of myocardial infarction. Arch Intern Med 118: 270–273.
Atar D, Bode C, Stuerzenbecher A, Verheugt FWA (2014). Anticoagulants for secondary prevention after acute myocardial infarction: lessons from the last decade. Fundamental & Clinical Pharmacology 28: 353–363.
Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R (1998). ISIS2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. BMJ 316: 1337–1343.
Bjerkelund CJ (1953). The method of administering dicoumarol. Lancet 1: 260–265.
Bjerkelund CJ (1957). The effect of long-term treatment with dicoumarol in myocardial infarction. A controlled clinical study. Acta Med Scand Suppl 330:1-212.
Borchgrevink CF (1960). Long-term anticoagulant therapy in angina pectoris and myocardial infarction. Acta Med Scand Suppl 359.
Bächli E (2000). History of tissue factor. British Journal of Haematology 110: 248–255.
Bønaa KH, Njølstad I, Ueland PM et al (2006). Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 354: 1578-88. doi: 10.1056/NEJMoa055227
Chalmers I, Matthews R, Glasziou P, Boutron I, Armitage P (2023). Analysis of clinical trial by Treatment Allocated or by Treatment Received? Applying ‘the intention-to-treat principle’. JLL Bulletin: Commentaries on the history of treatment evaluation. https://www.jameslindlibrary.org/articles/analysis-of-clinical-trial-by-treatment-allocated-or-by-treatment-received-applying-the-intention-to-treat-principle/.
Chalmers TC, Matta RJ, Smith H, Kunzler A-M (1977). Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. New England Journal of Medicine 297:1091-96.
Cohen J (1962). The statistical power of abnormal – social psychological research: A review. Journal of Abnormal and Social Psychology 65: 145–153.
Connors JM (2018). Testing and monitoring direct oral anticoagulants. Blood 132: 2009–2015.
Conrad LL, Kyriakopoulos JD, Wiggins CW, Honick GL (1964). Prevention of recurrences of myocardial infarction. Arch Intern Med 114: 348–358.
Corbett MS, Watson J, Eastwood A (2016). Randomised trials comparing different healthcare settings: an exploratory review of the impact of pre-trial preferences on participation, and discussion of other methodological challenges. BMC Health Serv Res 16: 589. doi: 10.1186/s12913-016-1823-6.
CURE (The clopidogrel in unstable angina to prevent recurrent events trial investigation) (2001). Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494–502.
Descôteaux J (2007). Statistical power: An historical introduction. Tutorials in Quantitative Methods for Psychology 3: 28–34.
Detry MA, Lewis RJ (2014). The Intention-to-Treat Principle. How to assess the true effect of choosing a medical treatment. JAMA 312: 85–86. doi:10.1001/jama.2014.7523
Eikelboom JW, Connolly SJ, Bosch J et al. (2017). Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 377: 1319-30. DOI: 10.1056/NEJMoa1709118
Enger E (1966). Kontrollerte kliniske forsøk [Controlled clinical trials]. Oslo: Universitetsforlaget.
European Society of Cardiology (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients
presenting with ST-segment elevation. European Heart Journal 39, 119–177. doi:10.1093/eurheartj/ehx393
European Society of Cardiology (2021). 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 42, 1289-1367. doi:10.1093/eurheartj/ehaa575
Fagerland MW, Lydersen S, Laake P (2015). Recommended confidence intervals for two independent binomial proportions. Statistical Methods in Medical Research 42: 224-254.
Franchini M, Liumbruno GM, Bonfanti C, Lippi G (2016). The evolution of anticoagulant therapy. Blood Transfusion 14: 175–184. doi: 10.2450/2015.0096-15
Hirsch J (2008). Antithrombotic therapy. In: 50 years of haematology. Research that revolutionized patient care. Washington DC: American Society of Hematology: 28–31.
Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H (2002). Warfarin, aspirin or both after myocardial infarction. N Engl J Med 347: 969–974.
International anticoagulant review group (1970). Collaborative analysis of long-term anticoagulant administration after acute myocardial infarction. Lancet 1: 203–209.
ISIS-2 (second international study of infarct survival) collaborative group (1988). Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2: 349–60.
Larsen Ø (2014). Doktorskole og medisinstudium. Det medisinske fakultet ved Universitetet i Oslo gjennom 200 år (1814–2014) [Doctoral school and medical studies. The medical faculty at the University of Oslo over 200 years (1814–2014)]. Michael Suppl 15: 598.
Life Insurance Companies’ Institute for Medical Statistics at the Oslo City Hospitals (1956). Myocardial infarction. An epidemiological and prognostic study of patients from five departments of internal medicine in Oslo 1935-1949. Acta Med Scand Suppl 315.
Loeliger EA (1981). Oral anticoagulation in the secondary prevention of myocardial infarction. Acta Med Scand Suppl 651: 305-315.
Montori VM, Guyatt GH (2001). Intention-to-treat principle. CMAJ 165: 1139–1141.
MoodleyJ, Naidoo S, Moodley J, Ramjee G (2016). Sharing of investigational drug among participants in the Voice Trial. AIDS Behav 20: 2709–2714. doi:10.1007/s10461-016-1414-x
Müller C (1938). Xanthomata, Hypercholesterolemia, Angina Pectoris. Acta Med Scand 95:75-84.
Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K (1968). A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. Scandinavian Journal of Clinical and Laboratory Investigation 21: suppl 105: 1-20. https://doi.org/10.1080/00365516809168196.
OASIS (The Organization to Assess Strategies for Ischemic Syndromes Investigators) (2001). Effects of Long-Term, Moderate-Intensity Oral Anticoagulation in Addition to Aspirin in Unstable Angina. J Am Coll Cardiol 37: 475–484.
Otterstad JE, Brosstad F (2007). Utviklingen av den medisinske behandlingen av hjertepasienter [The development of the medical treatment of cardiac patients]. In: Forfang K, Rasmussen K ed. Det norske hjerte. Norsk hjertemedisinsk historie. [The Norwegian Heart. Norwegian cardiology history]. Oslo: Universitetsforlaget: 193–205.
Owren PA (1947a). Parahaemophilia; haemorrhagic diathesis due to absence of a previously unknown clotting factor. Lancet 1: 446–444.
Owren PA (1947b). The coagulation of blood: investigations on a new clotting factor. Oslo: JC Gundersen.
Owren PA (1953). Long-term dicoumarol therapy in cardiovascular diseases. Acta Med Scand Suppl 287:46–48.
Owren PA, Hellem AJ, Ödegaard A (1964). Linolenic acid for the prevention of thrombosis and myocardial infarction. Lancet 284: 975–979.
Pedersen T (1985). Six-year follow-up of the Norwegian Multicenter Study on Timolol after acute myocardial infarction N Engl J Med 313: 1055-8. doi: 10.1056/NEJM198510243131705.
Pedersen T, Kjekshus J, Berg K et al. (1994). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383-1389.
Smith P, Arnesen H, Holme I (1990). The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med 323: 147–152.
Stormorken H (2000). Paul Arnor Owren. En medisinens mester [Paul Arnor Owren. A Master of Medicine]. Asker: Tell forlag.
Stormorken H (2003). The discovery of factor V: a tricky clotting factor. Journal of Thrombosis and Haemostasis 1: 206–13.
Strøm A, Jensen RA (1951). Mortality from circulatory diseases in Norway 1940–1945. Lancet 1: 126–129.
Timson DJ (2017). Dicoumarol: A drug which hits at least two very different targets in Vitamin K metabolism. Curr Drug Targets 18: 500–510. doi:10.2174/1389450116666150722141906
Wadsworth D, Sullivan E, Jacky T, Sprague T, Feinman H, Kim J (2021). A review of indications and comorbidities in which warfarin may be the preferred oral anticoagulant. J Clin Pharm Ther 46: 560–570. https://doi.org/10.1111/jcpt.13343
Wright IS, Marple CD, Beck DR (1948). Report of the committee for the evaluation of anticoagulants in the treatment of coronary thrombosis with myocardial infarction. American Heart Journal 36: 801–815.
Nylenna, M (2023). Paul Owren, Christopher Bjerkelund and the dawn of controlled trials in Norway.
© Magne Nylenna, Institute of Health and Society, University of Oslo, P.O. Box 1130 Blindern, 0318 Oslo, Norway. Email: magne@nylenna.no.
Cite as: Nylenna, M (2023). Paul Owren, Christopher Bjerkelund and the dawn of controlled trials in Norway. JLL Bulletin: Commentaries on the history of treatment evaluation (https://www.jameslindlibrary.org/articles/paul-owren-christopher-bjerkelund-and-the-dawn-of-controlled-trials-in-norway/)
Paul Arnor Owren (1905–1990)
In 1943, during the German occupation of Norway, a 29-year-old woman was admitted to Medical Department A at The National Hospital (Rikshospitalet) in Oslo. She would become crucial to a tradition of coagulation research in Norway after World War II. The patient had had prolonged nosebleeds, severe menorrhagia (menstrual bleeding), and other signs of a bleeding disorder. As the patient was a woman, classical hemophilia was out of the question so a search for the reason for her symptoms began (Stormorken 2003).
The patient’s physician was Paul Arnor Owren, a young doctor who had graduated from Medical School at The University of Oslo in 1931. After some years working as a general practitioner in Lillehammer he left his practice and became a fox farmer, to provide fox pelts for the clothing industry (!). Owren even published a few papers on insemination in foxes. However, in 1939 he returned to medicine and The National Hospital (Stormorken 2000) (Figure 1).
Figure 1. Professor Helge Stormorken (1922–2019) published a biography of Paul Owren in 2000 (Stormorken 2000).
Owren found the woman with bleeding of particular interest. With only scarce resources, he began to investigate her coagulation system. The University of Oslo had been closed by the German occupiers, so the prerequisites for research were limited. Furthermore, Owren had no previous academic training. Despite these obstacles, based on investigating his patient, Owren succeeded in identifying a new clotting factor. He named it Factor V because, since 1905, Paul Morawitz (1879–1936) had already identified four clotting factors (Bächli 2000).
In 1944, Owren presented his findings at a meeting of The Norwegian Academy of Science and Letters, and in 1946, at the XVII International Congress of Physiology in Oxford. An account of his research was published in The Lancet in 1947 (Owren 1947a), and he defended a doctoral thesis based on his research later that year (Owren 1947b).
Paul Owren became head of the Medical Department A at The National Hospital and full professor at the University of Oslo in 1949 – a position he held for the following 20 years. He continued his research and delivered important contributions to understanding the mechanisms of coagulation. He was the first to publish a case series of long-term dicoumarol therapy in Norway (Owren 1953). He also developed a method for monitoring anticoagulation therapy (“Thrombotest”) that made him both famous and wealthy. He became a world-renowned researcher on coagulation and established his own research group and later an Institute for Thrombosis Research. This attracted scores of talented researchers: a generation of Norwegian internists and hematologists obtained their scientific training at “Stall Owren”.
Christopher Juel Bjerkelund (1916–2002)
One of the many young doctors whose medical careers began under Owren’s leadership was Christopher Juel Bjerkelund (Figure 2). Bjerkelund graduated from the Medical School at the University of Oslo in 1945 and he held short hospital posts in Trondheim and Kristiansand before beginning his training as an internist at Medical Department A at The National Hospital in 1947. In the preface of his thesis ten years later he wrote: ”The basic knowledge of blood coagulation and anticoagulant therapy which I was taught as an assistant and research fellow in [Owren’s] department and laboratory was a necessary prerequisite for this investigation” (Bjerkelund 1957)
Figure 2. Christopher Bjerkelund married Agnes (née Riiber), a nurse, in 1944. They had one child, Carl Eivind (b. 1949). Photo: Private, reproduced with permission from Agnes and Carl Eivind Bjerkelund.
In 1950, Bjerkelund moved to Department VIII at Ullevål Hospital, a municipal hospital in Oslo, and trained there as a cardiologist under Professor Carl Müller (1886–1983), well known because of his research on familial hypercholesterolaemia (Müller-Harbitz’ disease) collaborating with the Norwegian pathologist Francis Harbitz (1867–1950) (Müller 1938). Bjerkelund remained at this department for 13 years and completed his doctoral thesis there in 1957 (Bjerkelund 1957). In 1963 he returned to The National Hospital as an assistant professor and consultant until 1969, when he became full professor and head of internal medicine at Aker Hospital, another municipal hospital in Oslo. He retired in 1986 and practiced privately in Oslo for many years.
Bjerkelund became a prominent figure in Norwegian cardiology in the 1950s. He was chair of the Norwegian Society of Cardiology and the Norwegian Society for Internal Medicine, and a Fellow of the American College of Cardiology. He was a member of the editorial board of The Journal of the Norwegian Medical Association from 1976 to 1988. As a former editor I remember him as a kind man, who could use a firm tone of voice when needed. His only child, Carl Eivind Bjerkelund (b. 1949), an anesthesiologist, describes his father as a decisive man who did not hesitate to participate in academic debates (CE Bjerkelund, personal communication, 15 May 2023). When, in the 1960s, Owren advocated the use of linolenic acid for the prevention of myocardial infarction (Owren et al. 1964), Bjerkelund was among the many who opposed his former boss, a position that was later confirmed in clinical studies (Natvig et al. 1968). Next to medicine, music, literature and the visual arts were Christopher Bjerkelund’s most important interests. He died in 2002 at the age of 86. At the time of writing this article (2023), Christopher Bjerkelund’s widow, Agnes Bjerkelund (b. 1920), is living alone in her own house at the age of 102.
Bjerkelund’s research on myocardial infarction
Before World War II, the incidence of myocardial infarction was increasing slightly in Norway but fell during the war (Strøm & Jensen 1951). Probably due to altered nutrition and readier access to tobacco there was a dramatic increase in incidence after the war, especially among middle-aged men. Among men in Oslo aged 40–59 years old there was a fourfold increase in the incidence of myocardial infarction between 1945 and 1950, from 4/10 000 per year to 16/10 000 (Life Insurance Companies’ Institute for Medical Statistics at the Oslo City Hospitals 1956).
Anticoagulant therapy for thromboembolic diseases had been introduced in the 1940s and vitamin-K antagonists were used for long-term treatment (Franchini et al. 2016). Dicoumarol, a drug with bioactive properties, was initially discovered during investigation of a mysterious disease of cattle. The drug was developed as a pharmaceutical product and replaced by warfarin in the 1950s (Timson et al. 2017). Due to the war, dicoumarol was not available in Norway until 1947 and warfarin was not approved by the Norwegian drug authorities until 1962.
Of Christopher Bjerkelund’s 53 entries in PubMed, a third are on myocardial infarction and/or anticoagulation. As a sign of the times in medical publishing, 32 of his papers were in Norwegian and only 21 in English. Bjerkelund had a special interest in the administration of dicoumarol and published a six-page article on this in The Lancet in 1953 (Bjerkelund 1953). His study on long term treatment with dicoumarol to prevent recurrences after myocardial infarction (Bjerkelund 1957) established a path for later Norwegian studies on this topic.
The title of Bjerkelund’s publication suggests that it was the first Norwegian thesis at the University of Oslo to be labelled a “controlled clinical trial” (Larsen 2014). It was published as a 212-page monograph and as a supplement to Acta Medica Scandinavica (from 1989, Journal of Internal Medicine) (Bjerkelund 1957)
Bjerkelund defended his thesis on 19 October 1957. His opponents were Paul Owren (Bjerkelund’s former boss) and Hans Jacob Ustvedt (1903–1982), who five years later left medicine to become head of the Norwegian Broadcasting Corporation.
Bjerkelund’s assessment of anticoagulant prophylaxis after myocardial infarction.
The increasing incidence of myocardial infarction after World War II led to an intensive search for treatment and preventive measures. Reports were published in 1948 on the benefit of anticoagulation with dicoumarol in the acute phase of myocardial infarction (Wright et al. 1948). Whether such treatment also had a prophylactic effect on recurrent heart attacks was unclear.
In a literature review in his thesis, Bjerkelund concluded that there were “no convincing statistical results available from carefully planned and controlled therapeutic trials”. “[A]rranging the best possible controlled clinical trial in which the course of the disease can be compared statistically in treated and untreated patients from the same period and the same source… has been the object and is the justification of this study” (Bjerkelund 1957, p 42).
Bjerkelund’s research question was: “Will continuous anticoagulant administration after acute myocardial infarction improve the prognosis in a given patient in relation to a similar patient without this form of treatment?” (Bjerkelund 1957, p 15).
Reduction in mortality, incidence of recurrent infarction and “perhaps a decrease in the number of thromboembolic complications” were his chosen endpoints.
Study design
As far as I am aware, Bjerkelund’s study was the first controlled clinical intervention study in Norway. Despite its possible biases and methodological flaws, it was an important precursor to later randomized controlled trials (RCTs).
Bjerkelund stated that the “factors affecting the prognosis should be as evenly distributed as possible between the two groups to be compared” (Bjerkelund 1957, p 43), and he discussed different ways of achieving this:
In the event he rejected both these methods because “it was obvious that one of the greatest difficulties (…) would be to keep the control group intact” (Bjerkelund 1957, p 44). The problem of drug sharing and patient preferences in randomized trials remains recognized as an issue today (Corbett et al. 2016; Moodley et al. 2016).
Bjerkelund had realized by 1957 that “Any new treatment is, in the eyes of the public, always the best, and there was reason to fear that, as the patients in the control group gradually came to know that some patients were treated in this way [with anticoagulation], they would want the same for themselves” (Bjerkelund 1957, p 44). Patients at that time were housed in wards with up to 22 beds and it was unavoidable that they would – during a month’s stay (which was common) – discuss their treatment and follow up. Bjerkelund feared that patients allocated to the control group would demand anticoagulation when they heard of the possibility. Therefore, he decided “on careful consideration” that it would be “best to let the question of to which group a patient was to be included would depend on to which department he had been admitted” (Bjerkelund 1957, p 45).
Ullevål Hospital had three departments of internal medicine (VII, VIII, IX). Admissions were handled by a hospital bed service outside the hospital, the choice of department being based on the number of empty beds. The treatment procedure for myocardial infarction was identical in the three departments, with anticoagulation prophylaxis during the first month after an acute infarction. In department VII anticoagulation would be terminated after one month and patients assigned to the control group. In department VIII long term anticoagulation therapy would continue with patients assigned to the intervention group. In department IX (the smallest of the three) patients would be assigned alternately either to the control group or to the intervention group for periods of about half a year.
Bjerkelund worked alone and was in personal charge of the follow-up of all patients included in the trial.
Patients in the intervention group had to attend follow-up assessments at intervals of a few weeks for their coagulation status to be monitored. Bjerkelund decided that follow-up and blood tests of those receiving placebo was impracticable and unethical, so they were assessed every 3–4 months.
In the Preface to his thesis Bjerkelund acknowledges Erling Sverdrup (1917–1994), a pioneer in Norwegian mathematical statistics, for “invaluable help and advice in the statistical investigation of my observations” (Bjerkelund 1957, p 5). No statistical power calculation was done to estimate the number of participants needed, but that would have been rare in the 1950s (Descôteaux 2007; Cohen 1962).
The study population
Patients under 76 years who had survived an acute myocardial infarction for at least one month were included. Six exclusion criteria were defined, before inclusion, for example, mental illness and contraindications to the use of anticoagulants.
The study population consisted of 277 patients with myocardial infarction. They were included from July 1950 to July 1953 and followed up to February 1956. 138 patients were assigned to the intervention group and 139 to the control group. The two groups were found to be similar regarding age, gender, social standing, and clinical status. Bjerkelund concluded that “the statistical comparison of the patients in the treated and control groups provides a good basis for stating that the patients were allotted by chance to the two groups” (Bjerkelund’s emphasis) (Bjerkelund 1957, p 87).
According to predetermined criteria, 12 patients were excluded from the intervention group and 14 from the control group before observation. I have constructed a flow chart of the study using Bjerkelund’s data (Figure 3).
Figure 3. Flow chart of the two groups recruited between July 1950 and July 1953 and observed until February 1956 produced by Magne Nylenna based on (Bjerkelund 1957).
Findings
During the observation period, a further 14 patients were excluded, 7 from each group. The reasons for dropout in the intervention group were 2 patients with serious bleeds, 1 with progressive dementia, and 4 who refused further participation. In the control group, 6 patients received long-term anticoagulation from other doctors, or for other reasons, and 1 refused to participate. Patients with short term anticoagulation during the observation period in the control group, or intermittent irregularities or interruptions (for example, for surgery) in anticoagulation in the intervention group, were included in the final analyses. Recurrences of myocardial infarction and mortality were analyzed according to timing of incidents and age of participants.
By February 1956, both the incidence of recurrences and the mortality were lower in the intervention group than in the control group. Bjerkelund reported statistically significant effects of anticoagulation were found among patients under 60 years of age during the first 12 months of treatment. Twenty-two patients in the intervention group had recurrent infarctions and 9 of them died, compared to 38 patients in the control group, of whom 21 died. A total of 24 deaths were registered in the intervention group (23 from cardiovascular disease) compared to 42 deaths in the control group (38 from vascular disease). Bjerkelund emphasizes that this does not mean “that mortality in the treated group is only 4/7 as large in the control group” (Bjerkelund 1957, p 140), but it is hard to understand the details of his statistical analyses and his use of expressions such as “force of recurrences” and “force of mortality”. Luckily this is not crucial to the importance of the study design.
A reanalysis of the raw data yields a statistically significant effect of anticoagulation on recurrences and mortality (Table 1).
Table 1. Reanalyses of raw data from Bjerkelund’s thesis (Bjerkelund 1957). 95% Confidence Intervals (CI) calculated using the Newcombe Hybrid score method (Fagerland et al. 2015). P-value calculated using the Pearson chi squared test.
Comments
Research method
Christopher Bjerkelund’s paper is first and foremost important because of the date (1957) it was published. Even with its shortcomings it is sophisticated for its time.
Bjerkelund was aware of the need for randomization in controlled clinical trials and of different ways of achieving this. His reason for leaving the responsibility for allotting patients to the two comparison groups based on hospital department admission to an external bed bureau instead of drawing lots was primarily to reduce the risk of losing “untreated” patients. After “careful consideration” he decided that the risk of many control patients demanding anticoagulation was more important than the disadvantages of suboptimal allocation. Despite these precautions 6 patients in the control group were given long-term anticoagulation and excluded from the final analyses.
This exclusion, as well as the exclusion of 7 dropouts from the intervention group, is a breach of the intention-to-treat principle as defined today. The true effect of an intervention, like anticoagulation in Bjerkelund’s study, is influenced by more than the biological effect of dicoumarol, for example, by variations in compliance, adherence to a treatment plan, and by other factors. Accordingly, all patients intended for treatment or control should be included in the final analysis (Detry and Lewis 2014; Montori and Guyatt 2001; Chalmers et al. 2023). The 7 dropouts in the intervention group should have been included in an intention-to-treat analysis. It is less clear how data relating to the 6 patients in the control group who were prescribed long-term anticoagulation should be handled. Bjerkelund specified that patients in the intervention group who had had periods without anticoagulation and patients in the control group who had periods with anticoagulation were to be included in the analysis. For this reason his study design should be characterized as a ‘modified intention-to-treat analysis’ (Detry et al. 2014; Chalmers et al. 2023).
Bjerkelund’s “careful consideration” does not meet today’s requirements for a randomized controlled trial, even though he discussed the limitations of his research openly. By mid-20th century there was more room for personal appraisal in all fields of medicine. “Clinical freedom” had not yet been replaced by procedures, guidelines and evidence-based medicine. Clinical research was not regulated by international conventions, ethics committees and requirements for informed consent.
By then the quality of research methods had already become a topic of heated discussions. One of my first informants for this paper was Christian Borchgrevink (b.1924) who worked with Owren in the late 1950s and early 1960s and knew Bjerkelund and his work well. When I erroneously referred to Bjerkelund’s thesis as a randomized trial Borchgrevink corrected me immediately, urging me to “remember, it was not a randomized trial. He [Bjerkelund] allocated patients by department, not by chance” (CF Borchgrevink, personal communication, 16 May 2023). In 1966, Peter Armitage and Christian Borchgrevink (Armitage and Borchgrevink 1966) strongly criticized a study reported two years earlier on the prevention of recurrences of myocardial infarction. Loyal L. Conrad et al. had written that past trials “have been designed so poorly that they do not qualify as controlled medical trials” (Conrad et al. 1964). Armitage and Borchgrevink in return accused Conrad et al. for “inappropriate statistical methods” and “inadequate number of patients” (Armitage and Borchgrevink 1966).
The first textbook on controlled trials in Norway was published in 1966 by Erik Enger (1927–2016). He stated that “Previous studies of anticoagulant therapy in this country have avoided placebo and the double-blind technique because of practical, ethical and medico-legal difficulties” (Enger 1966 p 99) (translated by MN). Forty years later, Bjerkelund’s trial and studies from the 1960s were commented as follows: “Based on today’s standards, many will probably claim that these studies were too small and with too short a follow-up to be able to demonstrate with certainty an effect on so-called “hard endpoints.” (Otterstad and Brosstad 2007) (translated by MN).
In an early example of a systematic review on anticoagulation in myocardial infarction, Thomas Chalmers (1917–1995) and his colleagues pointed out “the poor quality of the papers from the nonrandom trials, which revealed the largest anticoagulant effect on case fatality rates, and the minimal differences between treatments in most of the random control trials” (Chalmers et al. 1977).
Christopher Bjerkelund (Bjerkelund 1957) and Christian Borchgrevink (Borchgrevink 1960) paved the way for a series of later Norwegian RCTs in preventive cardiology conducted according to today’s strict requirements. Terje Pedersen (b. 1945) was principal investigator for the studies of timolol (Pedersen 1985) and simvastatin (Pedersen et al. 1994). Harald Arnesen (b.1938) led the two Norwegian Warfarin Re-Infarction Studies (WARIS) published in 1990 and 2002 (Smith et al. 1990; Hurlen et al. 2002). Kaare H. Bønaa (b. 1952) was principal investigator in the NORVIT-trial assessing the effect of lowering homocysteine (Bønaa et al. 2006).
Anticoagulation
Bjerkelund’s findings in 1957 were in line with other contemporary studies. In an analysis of nine controlled trials of long-term anticoagulation after myocardial infarction of which Bjerkelund’s was the first, mortality was 20 % lower in men in the intervention group than in controls (International Anticoagulant Review Group 1970). The review group could not, however, reach agreement on clinical recommendations which, in a review in 1981 (also including Bjerkelund’s study) (Loeliger 1981), was seen as the main reason for abandoning routine oral anticoagulation. The use of oral anticoagulants remained controversial during the last two decades of the 20th century despite studies that showed a beneficial effect (Smith et al. 1990; Anand and Yusuf 1999; Hurlen et al. 2002). The risk of bleeding complications was one of the main objections.
Direct oral anticoagulants (DOACs), replacing traditional vitamin K antagonists, have improved the safety of anticoagulation significantly (Connors 2018), but other long-term treatments have limited the indication for anticoagulant drugs in coronary heart disease.
By the turn of the century antiplatelet therapy had become a game changer (Hirsch 2008). Beneficial effects of acetylsalicylic acid (aspirin) in myocardial infarction had already been documented in 1988 (ISIS-2 1988) and suggested a survival advantage of up to ten years (Baigent et al. 1998). Adding oral anticoagulation to aspirin seemed to have little additional effect (OASIS 2001). The introduction of new antiplatelet agents such as clopidogrel (CURE 2001) established an undisputed requirement of dual antiplatelet therapy (aspirin + a P2Y12 inhibitor). A combination of one particular DOAC (rivaroxaban) and aspirin (Eikelboom et al. 2017) was termed a “dual-pathway” alternative (Atar et al. 2014) and showed clinical efficacy.
According to the pertinent European Society of Cardiology Guidelines (2018; 2021), antiplatelet therapy is now the first choice for preventing recurrences after myocardial infarction. The extensive use of percutaneous coronary intervention (PCI) and intracoronary stent implantation in acute coronary syndromes demands effective antiplatelet therapy to prevent stent thrombosis. This explains to a great extent the shift from anticoagulation to antiplatelet therapy following myocardial infarction. The patients included in Bjerkelund’s study differ from post-infarction patients today with coronary artery stents and reduced infarct size. A remaining indication for anticoagulation is left ventricular thrombus following a large infarction. However, contemporary treatment includes effective reperfusion therapy in the acute phase to reduce infarct size and thus the risk of thrombus formation in the ventricle.
Today the main indications for oral anticoagulation are atrial fibrillation/ prevention of stroke, venous thromboembolism, pulmonary embolism, and status after heart valve replacement (Altiok and Marx 2018; Wadsworth et al. 2021).
Conclusion
Christopher Bjerkelund was undoubtedly ahead of his time, both in clinical research and in the use of anticoagulation. According to his obituary (Abildgaard and Orning 2002), just before he died, Bjerkelund saw the beneficial effects of long-term anticoagulation after myocardial infarction confirmed in the Norwegian WARIS II-study as a feather in his cap (Hurlen et al. 2002).
Acknowledgements
I am grateful to Stian Lydersen, Professor of Medical Statistics at the Norwegian University of Science and Technology, for his contribution to statistical analyses. I am also grateful to Professor Erlend Hem for help with literature searches, and to Professor Dan Atar, Professor Chr. Borchgrevink, Professor Dag Thelle, Professor Rune Wiseth, Dr Carl Eivind Bjerkelund and Dr Iain Chalmers for valuable contributions.
This James Lind Library article has been republished in the Journal of the Royal Society of Medicine 2024;117:77-84. Print PDF
References
Abildgaard U, Orning O (2002). Christopher Juel Bjerkelund. Aftenposten 16 July 2002.
Altiok E, Marx N (2018). Oral Anticoagulation Dtsch Arztebl Int 115: 776–783.
Anand SS, Yusuf S (1999). Oral anticoagulant therapy in patients with coronary artery disease: A meta-analysis. JAMA 282: 2058–2067.
Armitage P, Borchgrevink CF (1966). Prevention of recurrences of myocardial infarction. Arch Intern Med 118: 270–273.
Atar D, Bode C, Stuerzenbecher A, Verheugt FWA (2014). Anticoagulants for secondary prevention after acute myocardial infarction: lessons from the last decade. Fundamental & Clinical Pharmacology 28: 353–363.
Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R (1998). ISIS2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. BMJ 316: 1337–1343.
Bjerkelund CJ (1953). The method of administering dicoumarol. Lancet 1: 260–265.
Bjerkelund CJ (1957). The effect of long-term treatment with dicoumarol in myocardial infarction. A controlled clinical study. Acta Med Scand Suppl 330:1-212.
Borchgrevink CF (1960). Long-term anticoagulant therapy in angina pectoris and myocardial infarction. Acta Med Scand Suppl 359.
Bächli E (2000). History of tissue factor. British Journal of Haematology 110: 248–255.
Bønaa KH, Njølstad I, Ueland PM et al (2006). Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 354: 1578-88. doi: 10.1056/NEJMoa055227
Chalmers I, Matthews R, Glasziou P, Boutron I, Armitage P (2023). Analysis of clinical trial by Treatment Allocated or by Treatment Received? Applying ‘the intention-to-treat principle’. JLL Bulletin: Commentaries on the history of treatment evaluation. https://www.jameslindlibrary.org/articles/analysis-of-clinical-trial-by-treatment-allocated-or-by-treatment-received-applying-the-intention-to-treat-principle/.
Chalmers TC, Matta RJ, Smith H, Kunzler A-M (1977). Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. New England Journal of Medicine 297:1091-96.
Cohen J (1962). The statistical power of abnormal – social psychological research: A review. Journal of Abnormal and Social Psychology 65: 145–153.
Connors JM (2018). Testing and monitoring direct oral anticoagulants. Blood 132: 2009–2015.
Conrad LL, Kyriakopoulos JD, Wiggins CW, Honick GL (1964). Prevention of recurrences of myocardial infarction. Arch Intern Med 114: 348–358.
Corbett MS, Watson J, Eastwood A (2016). Randomised trials comparing different healthcare settings: an exploratory review of the impact of pre-trial preferences on participation, and discussion of other methodological challenges. BMC Health Serv Res 16: 589. doi: 10.1186/s12913-016-1823-6.
CURE (The clopidogrel in unstable angina to prevent recurrent events trial investigation) (2001). Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494–502.
Descôteaux J (2007). Statistical power: An historical introduction. Tutorials in Quantitative Methods for Psychology 3: 28–34.
Detry MA, Lewis RJ (2014). The Intention-to-Treat Principle. How to assess the true effect of choosing a medical treatment. JAMA 312: 85–86. doi:10.1001/jama.2014.7523
Eikelboom JW, Connolly SJ, Bosch J et al. (2017). Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 377: 1319-30. DOI: 10.1056/NEJMoa1709118
Enger E (1966). Kontrollerte kliniske forsøk [Controlled clinical trials]. Oslo: Universitetsforlaget.
European Society of Cardiology (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients
presenting with ST-segment elevation. European Heart Journal 39, 119–177. doi:10.1093/eurheartj/ehx393
European Society of Cardiology (2021). 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal 42, 1289-1367. doi:10.1093/eurheartj/ehaa575
Fagerland MW, Lydersen S, Laake P (2015). Recommended confidence intervals for two independent binomial proportions. Statistical Methods in Medical Research 42: 224-254.
Franchini M, Liumbruno GM, Bonfanti C, Lippi G (2016). The evolution of anticoagulant therapy. Blood Transfusion 14: 175–184. doi: 10.2450/2015.0096-15
Hirsch J (2008). Antithrombotic therapy. In: 50 years of haematology. Research that revolutionized patient care. Washington DC: American Society of Hematology: 28–31.
Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H (2002). Warfarin, aspirin or both after myocardial infarction. N Engl J Med 347: 969–974.
International anticoagulant review group (1970). Collaborative analysis of long-term anticoagulant administration after acute myocardial infarction. Lancet 1: 203–209.
ISIS-2 (second international study of infarct survival) collaborative group (1988). Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 2: 349–60.
Larsen Ø (2014). Doktorskole og medisinstudium. Det medisinske fakultet ved Universitetet i Oslo gjennom 200 år (1814–2014) [Doctoral school and medical studies. The medical faculty at the University of Oslo over 200 years (1814–2014)]. Michael Suppl 15: 598.
Life Insurance Companies’ Institute for Medical Statistics at the Oslo City Hospitals (1956). Myocardial infarction. An epidemiological and prognostic study of patients from five departments of internal medicine in Oslo 1935-1949. Acta Med Scand Suppl 315.
Loeliger EA (1981). Oral anticoagulation in the secondary prevention of myocardial infarction. Acta Med Scand Suppl 651: 305-315.
Montori VM, Guyatt GH (2001). Intention-to-treat principle. CMAJ 165: 1139–1141.
MoodleyJ, Naidoo S, Moodley J, Ramjee G (2016). Sharing of investigational drug among participants in the Voice Trial. AIDS Behav 20: 2709–2714. doi:10.1007/s10461-016-1414-x
Müller C (1938). Xanthomata, Hypercholesterolemia, Angina Pectoris. Acta Med Scand 95:75-84.
Natvig H, Borchgrevink CF, Dedichen J, Owren PA, Schiotz EH, Westlund K (1968). A controlled trial of the effect of linolenic acid on incidence of coronary heart disease. Scandinavian Journal of Clinical and Laboratory Investigation 21: suppl 105: 1-20. https://doi.org/10.1080/00365516809168196.
OASIS (The Organization to Assess Strategies for Ischemic Syndromes Investigators) (2001). Effects of Long-Term, Moderate-Intensity Oral Anticoagulation in Addition to Aspirin in Unstable Angina. J Am Coll Cardiol 37: 475–484.
Otterstad JE, Brosstad F (2007). Utviklingen av den medisinske behandlingen av hjertepasienter [The development of the medical treatment of cardiac patients]. In: Forfang K, Rasmussen K ed. Det norske hjerte. Norsk hjertemedisinsk historie. [The Norwegian Heart. Norwegian cardiology history]. Oslo: Universitetsforlaget: 193–205.
Owren PA (1947a). Parahaemophilia; haemorrhagic diathesis due to absence of a previously unknown clotting factor. Lancet 1: 446–444.
Owren PA (1947b). The coagulation of blood: investigations on a new clotting factor. Oslo: JC Gundersen.
Owren PA (1953). Long-term dicoumarol therapy in cardiovascular diseases. Acta Med Scand Suppl 287:46–48.
Owren PA, Hellem AJ, Ödegaard A (1964). Linolenic acid for the prevention of thrombosis and myocardial infarction. Lancet 284: 975–979.
Pedersen T (1985). Six-year follow-up of the Norwegian Multicenter Study on Timolol after acute myocardial infarction N Engl J Med 313: 1055-8. doi: 10.1056/NEJM198510243131705.
Pedersen T, Kjekshus J, Berg K et al. (1994). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383-1389.
Smith P, Arnesen H, Holme I (1990). The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med 323: 147–152.
Stormorken H (2000). Paul Arnor Owren. En medisinens mester [Paul Arnor Owren. A Master of Medicine]. Asker: Tell forlag.
Stormorken H (2003). The discovery of factor V: a tricky clotting factor. Journal of Thrombosis and Haemostasis 1: 206–13.
Strøm A, Jensen RA (1951). Mortality from circulatory diseases in Norway 1940–1945. Lancet 1: 126–129.
Timson DJ (2017). Dicoumarol: A drug which hits at least two very different targets in Vitamin K metabolism. Curr Drug Targets 18: 500–510. doi:10.2174/1389450116666150722141906
Wadsworth D, Sullivan E, Jacky T, Sprague T, Feinman H, Kim J (2021). A review of indications and comorbidities in which warfarin may be the preferred oral anticoagulant. J Clin Pharm Ther 46: 560–570. https://doi.org/10.1111/jcpt.13343
Wright IS, Marple CD, Beck DR (1948). Report of the committee for the evaluation of anticoagulants in the treatment of coronary thrombosis with myocardial infarction. American Heart Journal 36: 801–815.